
WQ-5

When a water sample is tested the testing lab provides a report listing the concentration of materials found in the sample. Since little or no water in nature is chemically pure, most reports will indicate the presence of some natural occurring compounds such as calcium, magnesium, carbonates and chlorides. Federal and state agencies have established guidelines to indicate those levels of contaminants - both naturally occurring and introduced pollutants - considered safe. They also have established levels above which a possible health risk for different uses may occur.
Chemists divide materials into two classes: organic and inorganic. The term organic refers to materials which contain carbon. Examples of organic compounds would include petroleum fuels, solvents, paint thinners and pesticides. Inorganic compounds are not derived from living sources, and in general don't contain carbon. Inorganics include lead, nitrate and chloride. Three exceptions to these general rules are carbonates, a compound found in essentially all Indiana ground water, carbide and cyanide. These three are considered inorganic even though they do contain carbon.
This bulletin addresses the interpretation of water test reports for inorganic compounds commonly found in Indiana's water supplies. The bulletin also outlines the potential health risks when acceptable levels are exceeded.
Federal and state agencies review short- and long-term animal exposure studies before labeling a material as hazardous. The studies are used to determine the level of exposure at which acute or chronic health problems may occur.
Acute problems are those which arise quickly and may include nausea, skin rash, dizziness or death. An example would be a farmer who becomes dizzy and nauseous after spilling a pesticide. Bacteria and viruses may also cause acute illness.
More common than acute effects and more difficult to detect, chronic problems result from long-term exposure to very small amounts of a contaminant. Chronic problems include cancer (carcinogenic), birth defects (teratogenic), organ damage and nervous system disorders. In general, if a material enhances the risk of cancer, special notice is given.
Under the Safe Drinking Water Act, the Environmental Protection Agency (EPA) has set limits for the concentration levels of certain contaminants found in drinking water. Most of the substances regulated by the EPA occur naturally in the environment and the food you eat. These levels are set for public drinking water supplies only. However, the levels serve as guidelines for private water supplies.
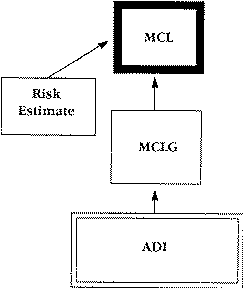
The EPA sets an Acceptable Daily Intake (ADI) level for all toxic materials. The ADI is the daily intake of a material a person can consume through food and water and not suffer adverse health effects, either acute or chronic. The ADI is an adjusted value. The minimum concentration, detertmined by research, to cause a health problem is further adjusted downward by a safety factor. The safety factor takes into account the uncertainties of applying research test results to humans and ensures that complying with the ADI levels will not result in any health problems.
The Maximum Contaminant Level Goal (MCLG) is the maximum level of a particular material a person should consume safely over a lifetime with no adverse health effects. The EPA does not enforce the MCLG. The ADI level, after adjustment for exposure only through drinking water, serves as the base for the MCLG.
The EPA uses the MCLG to set the Maximum Contaminant Level (MCL). The MCL is the maximum level of a material the EPA and state agencies allow in public drinking water supplies. When setting the MCL, regulators consider health risks, the cost of analysis and treatment limits. Because of these considerations, MCLs tend to be less strict (higher concentrations) than MCLGs.
A material considered a carcinogen or a teratogen has a MCLG of zero. However, a zero level is difficult to achieve and to monitor. Therefore, enforceable limits are set using a mathematical procedure to calculate a Risk Estimate. The risk estimate is based on the possibility that exposure, at some level, will cause one additional cancer per 100,000 or one million people over a lifetime of 70 years. EPA generally sets an acceptable (negligible) risk level at one in a million.
The difference between the ADI (Acceptable Daily Intake) method and the risk estimate method is that the ADI method assumes a safe minimum level (threshold value) exists for the compound. A risk estimate can be made for materials that cause some problem whenever the compound is found. The risk estimate is used to establish the Maximum Contaminant Level (MCL) in drinking water supplies. Thus drinking water standards are set up to minimize risk from using that resource. Figure 1 shows how the EPA levels are related.
(units used to report concentration in the sample)
In general, contaminant concentrations are reported in either milligrams per liter (mg/l) which equals parts per million (ppm) or micrograms per liter (ug/l) which equals parts per billion (ppb) (see Table 1). In both cases the numbers are small and represent the proportion of the contaminant in a million or billion parts of water. For example, to get a one ppb metal concentration you would dissolve one ounce of the metal into 1,000,000,000 ounces or 7,800,000 gallons of water. To arrive at ppm you would need to dissolve one ounce in 1,000,000 ounces or 7,800 gallons of water.
Hardness of water may be reported as grains per gallon (gpg). Hardness as gpg can be converted to ppm using the following equation:
Table 1. Units Used in Reporting Concentrations | |
|---|---|
| Parts Per Million | Parts Per Billion |
| milligrams per liter | micrograms per liter |
| (mg/l) | (ug/l) |
| 1 ppm = 1000 ppb | |
(acceptable limits in brackets)
pH indicates the acidity or alkalinity of water. An acidic pH, less than 6.5, causes metals to corrode and dissolve from pipes, fixtures or pumps. A pH of less than 4.5 indicates some type of mineral acid, such as from mine drainage, in the water. A basic pH, over 8.5, makes water feel slippery, leaves scaly deposits or causes water to have a soda taste.
Distilled water left in an open container to equilibriate with the air will have a pH of 5.5 to 5.7 due to carbon dioxide. Dissolved calcium and magnesium cause most ground water in Indiana to have a pH of 6.5 to 7.5.
Hard water contains calcium and magnesium salts. Hardness of Indiana ground water is generally between 20 to 400 ppm. Hardness does not impart a negative health effect. However, when water is heated calcium and magnesium salts fall out of solution and form scale on pans and in plumbing, coffee pots and water heaters. Hard water also requires extra soap in the laundry and makes glasses spot in the dishwasher. Typical ranges for water hardness are given in Table 2.
The general recommendation is water with hardness greater than 180 ppm should be conditioned with a water softener. Most softeners exchange calcium and magnesium for sodium, but they are designed only to remove hardness, not other chemicals.
| Table 2. Hardness Scale | |
| Classification | Range of hardness (ppm) |
| Soft | 0 - 60 |
| Moderately Hard | 61 - 120 |
| Hard | 121 - 180 |
| Very Hard | >180 (virtually all of Indiana's ground water is this range) |
Ground water supplies in Indiana typically have between 10.0 to 50.0 ppm chloride. Chlorides in water are generally not considered a health problem. At levels greater than 500.0 ppm, chlorides make water taste salty. At these levels, there can be accelerated corrosion of water heaters and plumbing fixtures. Very high levels may indicate some type of contamination typically from deicing salts, human sewage or animal manures, or industrial sources.
In Indiana, ground water can contain between 0.0 and 1,000.0 ppm sulfate. Sulfates of calcium and magnesium can cause hardness in water. Sulfate levels at 500.0 ppm or greater can have a laxative effect and cause an astringent aftertaste to the water. High sulfate levels can also have a corrosive effect on plumbing.
Water containing sulfate may also contain bacteria which produce hydrogen sulfide. The foul, rotten egg smell found in some water comes from hydrogen sulfide. Hydrogen sulfide is a poisonous gas but at levels dissolved in water, is not a health hazard. However, dissolved hydrogen sulfide causes silver and aluminum utensils to tarnish. Hydrogen sulfide is best detected with the nose (0.05 ppm is detectable) as it readily vaporizes from standing water.
Iron originates in soils and rocks, occurs naturally in water and is needed in human and animal diets. Iron in Indiana ground water spans a typical range from 0.1 to 3.0 ppm. At high concentrations (more than 0.3 ppm) iron will discolor (reddish-orange; brown-black) household fixtures, laundry and give an objectionable taste and odor to water. However, even at concentrations far over 0.3 ppm few adverse health effects have been reported. Bacteria which feed on iron can create an objectionable odor in the water and discharge a clear, oil-like slime, typically noticed in toilet tanks.
Manganese ranges from 0.02 to 1.0 ppm in Indiana ground water. At levels greater than 0.05 ppm manganese tends to fall out of solution and form black flakes. These flakes will deposit themselves in the same way iron stains and can clog pipes.
The range for fluoride in Indiana ground water is typically 0.1 to 1.5 ppm. At very high levels fluoride is toxic to humans. Water supplies seldom reach these levels. At levels between 0.7 and 1.2 ppm, fluoride will prevent tooth decay and is essential to proper development of bones and teeth. At levels greater than 4.0 ppm, fluoride may cause dental problems, including brownish discoloration. At levels greater than 6 ppm, skeletal problems may occur.
Besides calcium, magnesium, iron and manganese, water can contain many other metals (Table 3). These metals enter water as it moves through the ground, which contains naturally occurring metals. While high levels of metals in ground water may arise naturally, the source is more likely from human contamination. Potential sources of metals in water include but are not limited to: improperly applied sludge, industrial processing facilities, poorly constructed landfills and chemical spills.
The EPA sets primary MCLs to guard against adverse health effects. Secondary standards are set for some metals. Secondary standards serve as a guideline for taste, odor, color or other aesthetic aspects which do not present a health risk.
| Table 3 National Primary And Secondary Drinking Water Standards | ||
|---|---|---|
| Primary MCL (mg/L) | Secondary MCL (mg/l) | |
| Arsenic | 0.05 | |
| Barium | 1.00 | |
| Cadmium | 0.01 | |
| Chromium | 0.05 | |
| Chloride | 250.00 | |
| Copper | 1.00 | |
| Fluoride | 4.00 2.00 | |
| Iron | 0.3 | |
| Lead | 0.05 | |
| Manganese | 0.05 | |
| Mercury | 0.002 | |
| Selenium | 0.01 | |
| Silver | 0.05 | |
| Sulfate | 250.00 | |
| Zinc | 5.00 | |
| USEPA Office of Drinking Water, June 1989 | ||
The health effects of long- or short-term exposure to these metals varies. In general, long-term exposure to low levels or short-term exposure to high levels results in damage to the kidneys or liver. The exceptions are lead and mercury which can impact the central nervous system as well as other organs. The amount of metal that must be ingested to achieve an effect varies widely. If your water test report shows high levels of a metal(s) discuss the health effects with a health professional. You should consider using an another source of drinking water or an appropriate water treatment device. You also need to locate the possible cause of contamination.
Nitrate, an anion (negative charge), is not adsorbed by soil and moves with infiltrating water. Of the inorganic contaminants found in water, nitrate receives the most attention. This is due to the fact nitrate is easy to detect and many natural sources are present in the environment.
A large amount of confusion exists over the way nitrate data is presented. Table 4 gives the conversion factors for the common methods used. You should note the nitrogen value used in the test report for your well water. For example a reading of nitrate (NO3) at 44.0 ppm is equivalent to 10.0 ppm nitrogen (NO3-N). NO3-N refers to nitrate by the amount of nitrogen present.
| Table 4. Expression of Nitrate and Nitrite Values | |||
|---|---|---|---|
| Method 2 | |||
| N | NO2 | NO3 | |
| Method 1 | |||
| Nitrate as Nitrogen (NO3-N) | 1.0 | 3.3 | 4.4 |
| Nitrite as Nitrogen (NO2-N) | 1.0 | 3.3 | 4.4 |
| Nitrate (NO3) | 0.23 | 0.74 | 1.0 |
| Nitrite (NO2) | 0.3 | 1.0 | 1.34 |
| Method 1 x Method 2 = type under 2 NO3 is 23% Nitrogen by weight | |||
While natural nitrate originating from soil occurs in water, (0.2 to 0.3 ppm NO3-N), high levels of nitrate in well water, like chloride, indicate surface contamination. These sources include: septic fields, manure pits and lagoons, and fertilizer and sludge application.
If the water contains more than 10.0 ppm NO3-N it may cause infant cyanosis (blue-baby) in children under the age of six months. The cyanosis can be fatal to both infants and small animals. The water should not be given to infants either directly or used in formulas. It is also recommended that children and adults should avoid long-term consumption of water with over 10.0 ppm NO3-N.
Your water test report may show levels of other inorganic materials within the acceptable limits. Although this may mean the water is safe for drinking, there may be unwanted odor, taste or color. Most of these problems can be corrected with a water treatment system. Refer to the WQ bulletin on water treatment systems and consult with a commercial distributor to select a water treatment system for your home.
Water can also become contaminated with organic chemicals, either naturally or from human activities. If your water test report lists contamination from an organic chemical, refer to WQ bulletin "Interpreting Water Test Reports Part Two: Organic Materials".
For further information on water testing or possible contamination suspected in your area, contact your local Health Department or county Cooperative Extension office. The following bulletins in the WQ series may also be helpful:
WQ Bulletin "Interpreting Water Test Reports Part Two: Organic Materials"
WQ 6 "Buying Home Water Treatment Equipment"
"Is Your Drinking Water Safe?" EPA 570/9-89-005. Office of Water (WH-550). United States Environmental Protection Agency.
EPA Safe Drinking Water Hotline: 800/426-4791
Report unknown contamination or objectionable taste, odor or color in a private well to: Indiana Department of Environmental Management (IDEM) Ground Water section 317/240-6216
This material is based upon work supported by the U.S. Department of Agriculture ture, Extension Service, under special project number 89-EWQI-1-9202.
Cooperative Extension work in Agriculture and Home Economics, state of Indiana, Purdue University, and U.S. Department of Agriculture cooperating; H. A. Wadsworth, Director, West Lafayette, IN. Issued in furtherance of the acts of May 8 and June 30, 1914. The Cooperative Extension Service of Purdue University is an affirmative action/equal opportunity institution.