AE-89

Introduction Types of Materials Used for Solar Heat Storage Advantages and Drawbacks of the Various Storage Materials How Phase-Change Materials Work in Solar Heat Storage Size and Type of Rocks Best for Heat Storage Type of Heat Transfer Medium to Use Determining the Size of Your Storage Facility Location of Your Storage Facility Importance of Storage Facility Configuration (Shape) Reducing Your Required Storage Volume Suggestions When Buying a Commercial Heat Storage Device Related Publications
No one needs to define for the average citizen the term "energy crunch". Our monthly fuel and utility bills are constant reminders of the cost of America's standard of living. And the "experts" warn that the crisis is here to stay.
Of the alternatives to conventional forms of energy, the one receiving most serious consideration--at least for home, farm and small business heating needs--is solar energy. Today, many new homes are being planned and constructed to accommodate solar heating systems. Various types of portable collectors and solar heating conversion packages are readily available on the retail market.
Unfortunately, too many perspective users of solar energy have too little information about some of the aspects of building or converting to a solar heating system. One area of inadequate or misinformation in particular (and a costly one it mistakes are made) is the storage of collected energy. The purpose of this publication, therefore, is to answer some basic questions about the proper selection and use of thermal storage devices.
Included in the publication are discussions of various heat storage materials and transfer media, and how to select the `right' one; size, location and shape of the storage device; and suggestions on shopping for such a device. Included are two worksheets (with examples)-one for determining how much heat storage you will likely need, and the other for finding out how much you might be able to cut costs by proper insulation. Listed at the end of this publication are available Purdue Extension publications that deal with related aspects of solar heating and energy conservation.
A number of materials will work as storage media in home, farm or small business solar heating systems; but only three are generally recommended at this time--rock, water (or water-antifreeze mixtures) and a phase-change chemical substance called Glauber's salt. These are the materials that most consistently meet the criteria for selecting a storage medium--namely, the ability (1) to deliver heat to its application points at a desirable temperature, and (2) to do it cheaply, based not so much on cost of the material as on cost of the total system and its maintenance.
Thus, there is no one `best' heat storage material; but rather each of the three has characteristics that might make it the most desirable under certain conditions.
Rocks
As a storage material, rocks are cheap and readily available, have good heat transfer characteristics with air (the transfer medium) at low velocities, and act as their own heat exchanger. Main disadvantages are their high volume-per-BTU-stored ratio compared to water and phase-change materials (which means a bigger heat storage area), and difficulties with water condensation and microbial activity. If the dew point of the air coming into the storage is higher than the rock temperature, the moisture in the air condenses on the rocks. Moisture and heat in the rock bed can lead to microbial growth.
Rock storage is the most reliable of the three storage systems because of its simplicity. Once the system is installed, maintenance is minimal and few things can decrease the performance of the storage.
Air solar collectors are usually used with rock storage devices. Since air collectors are cheaper and more maintenance-free than liquid collectors, a system using rock storage and air solar collectors seems the most logical for residential heating. However, other circumstances, such as availability of cheap materials, limited collector or storage space or incompatibility with the existing heating system, may dictate the use of a water or phase-change material storage device. Remember, however, that the ultimate deciding factor should be the initial and maintenance costs of the system.
The type and size of rocks best tor heat storage is discussed later.
Water
Water as a storage material has the advantages of being inexpensive and readily available, of having excellent heat transfer characteristics, and of being compatible with existing hot water systems. Its major drawbacks include difficulties with system corrosion and leakage, and more expensive construction costs.
Because of the good heat-storage-to-volume ratio (five times greater than rock) and greater efficiency of liquid solar collectors, liquid collection and storage systems can be very practical: (1) where close maintenance is available (such as in multiple-residence or industrial buildings), (2) where the ultimate use is hot water (such as in a dairy barn or food processing facility), or (3) where the water storage system can be directly coupled with an existing heating system as in residential hot water baseboard heat.
A water storage system might also be considered instead of rock storage in situations where space is limited. The water tank can easily be buried below ground to save space.
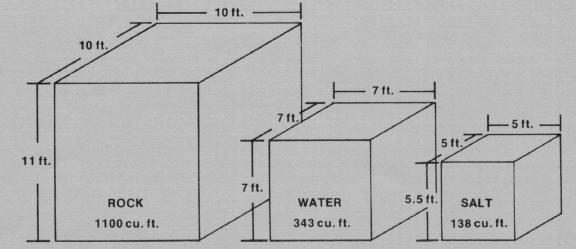
The phase-change material Glauber's salt, because of its low ratio of volume-per-BTU-stored, requires only 1/8 the space of rocks and 2/5 the space of water for comparable heat storage (see Figure 1). It also absorbs and releases most of its heat at a constant temperature. Disadvantages of Glauber's salt, at this point at least, are its cost relative to rock and water, and various technical problems (e.g., packaging problems due to its poor thermal conductivity and its corrosive nature). Such problems need to be resolved before the reliability of PCM's can be assured.
Phase-change materials are most generally used in situations where space limitations exist. Often times, the cost of additional space in a new home for a rock storage device will be greater than the added cost of buying a PCM such as Glauber's salt. These materials are also highly desirable where a premium is placed on maintaining a constant temperature. Living spaces heated by a PCM are often more comfortable, since the air temperature from the storage is more uniform while it is discharging.
PCM's are chemical substances that undergo a solid-liquid transition at temperatures within the desired range for heating purposes. During the transition process, the material absorbs energy as it goes from a solid to a liquid and releases energy as it goes back to a solid. What makes a PCM desirable for heat storage is its ability to hold greatly varying amounts of energy at the same temperature.
To illustrate, consider the phase changes that occur with water. If water is put in a freezer, heat is withdrawn from it by the refrigerant until it becomes ice. If the ice is then placed in a liquid at room temperature, it melts as it absorbs energy from that surrounding liquid. The amount of heat absorbed is about 143 BTU per pound, which means a pound of ice can cool a pound of water from 175°F down to 32°F while, itself, only changing form (i.e., from ice at 32° to water at 32°).
Currently being studied as potential heat storage materials are at least a dozen chemical compounds that change phase at temperatures within the useful range for space heating. However, at this point, only Glauber's salt (sodium sulfate decahydrate) is being sold commercially. Glauber's salt changes phases at 90°F and has a 108-BTU-per-pound "latent heat" (amount of heat absorbed or released during phase change). Because of its high latent heat, Glauber's salt requires less storage volume than either rock or water; that could mean lower storage facility cost and more usable space within the home to offset the material's relatively high cost.
PCM's do have some chemical traits that can present problems in heat storage and transfer; but most have been or are being overcome. One is that PCM's tend to overcool as heat is withdrawn. This means that, rather than giving up its latent heat at the phase-change temperature, salt PCM's may remain a liquid until they fall to possibly 15-30° below that temperature. To combat this super cooling" in Glauber's salt, about 3 percent of the chemical, sodium tetraborate decahydrate, is added to induce phase change at the proper temperature.
Another problem with salt PCM's is that of incongruent melting, which occurs when the salt is partially insoluble in the water of crystallization. In the case of Glauber's salt, at its melting temperature, about 15 percent of the sodium sulfate remains in insoluble anhydrous form. Being twice as dense as the saturated solution, the anhydrous settles out and will not recrystallize when heat is withdrawn. To prevent this, a thickening agent is used to keep the an hydrous in suspension until it can reform in the crystal structure when heat is removed.
The heat storage capability will drop from 108 to about 60 BTU per pound as the anhydrous settles out. Presently, the best thickening agent used is an attapulgite clay which, when added to the Glauber's salt in amounts of 7-10 percent, prevents settling out of the anhydrous and does not degrade with time.
Note: Beware of mixtures containing cellulose, starch, sawdust, silica gel, silica, etc. These types of thickening agents work well for a while, but eventually are either hydrolyzed by the salt or decomposed by bacteria and become ineffective. Dealing with a reputable company should eliminate some of these worries. Don't let a salesman sell you on a "secret" thickening agent; if it were any good it would be patented, and there would be no need for secrets.)
Although size of rock selected will be determined primarily upon cost, in general, the larger the size the better for storage purposes. The main reason is that it takes less power to force the heat transfer air through large stones than through small ones. Rocks less than an inch in diameter are usually too small; whereas ones more than 4-6 inches in diameter are too large, because of insufficient heat transfer surface area.
If gathering your own rock for storage, look for roundish field stones in the 4- to 6-inch diameter range. If buying commercially from a stone quarry, the largest size available is probably "septic gravel", which is 1-3 inches in diameter. But don't be overly concerned about size; settle for a 2-inch septic gravel if you'd have to pay a premium for larger rock. If available, old house brick is a good storage material when stacked to permit air flow.
Probably more important than rock size is uniformity of size. If there is too much variation, the smaller stones will fill in the voids between the larger stones, thus increasing the air blower power requirement. Also, avoid those types of rock that tend to scale and flake, such as limestone. The resulting "dust" is picked up by the heat transfer air and either clogs the furnace filters or, if the furnace is bypassed, is blown directly into the heating area.
Since air must be blown through the rock bed, it's necessary to know the amount of power needed. In general, the faster the air flow and/or the smaller the rock size, the greater the power requirement.
For example, an air velocity of 50 feet per minute through a 10-foot long bed of 1-inch rock has a pressure drop of about 1 inch water (static pressure). Decreasing the velocity to 30 feet per minute would cut the pressure drop to 1/2 inch water. The pressure drop across a total system (i.e., collector, storage bed and ductwork) should be no more than 3-4 inches water (static pressure).
Before filling the storage facility, consider washing or screening out "fines" which might otherwise fill in the voids. The rock storage should allow some way for accumulated moisture to be discharged. Also, consider ways to prevent mold and bacteria growth, one of which is keeping the storage temperature high even during periods of low use.
The transfer media most generally used in solar heating systems are air, water and water-antifreeze mixtures. Which one you should use might well be dictated by the type of storage material selected. For example, rock storage requires air as the transfer medium; water or water-antifreeze storage utilize the same liquid for heat transfer; PCM storage. on the other hand, would use either air or liquid, depending on the type of heat exchanger.
Many of the early solar homes constructed utilized water collectors with water storage because of the advantages of increased efficiency and reduced size. However, at present, solar heating systems that use air as the transfer medium are being recommended for home usage. One reason is less potential for damage; a faulty air transfer system would not cause nearly the problems that a leaky or frozen water system would. Also air collectors and ducting are usually cheaper and require less maintenance. Until more reliable and "fail safe" liquid systems are developed, air will likely continue to be the recommended transfer medium for home solar heating.
The volume of storage needed depends on four factors-(1) heating requirement of the area to be heated, (2) days of storage reserve desired, (3) temperature range over which the heat is stored, and (4) type of storage material used. Following is a brief discussion of each factor and Worksheet I (with example) for calculating needed heat storage capacity using different storage materials.
Heating requirement is the heat needed to maintain a desired temperature in the home or other building. It is equal to the amount of heat that the structure loses to the surroundings through its walls and roof by heat conduction and convection. This heat loss can be estimated by following the simple equations found in most heat transfer books (see Related Publications on page 9) or often, gas and heating company representatives will make such determinations as a service.
Storage reserve is the amount of heat needed if energy cannot be collected for a given number of days. Although quite variable, the amount of reserve generally planned for in home solar heating at present is 3 to 5 days.
Temperature range over which the heat is stored is the difference between the maximum temperature of the storage bed when full and minimum temperature that the heat transfer fluid should be for heating. In solar-heated homes, maximum "bed" temperature is likely to be 130-150°F, depending on the collector used; whereas minimum transfer temperature is about 75-80°F, assuming a desired room temperature of 70°F. Thus. a good "temperature range" figure to use in storage volume calculations would be 50°F (130° - 80°) (There is a tendency to store heat at the highest possible temperature to minimize size of the storage facility; but as the temperature from the collector rises, the efficiency falls).
Heat storage materials differ in certain characteristics which also must be considered in determining storage capacity. Table 1 lists the bulk density, specific heat (thermal capacity) and latent heat of the three common solar heat storage materials--rock, water and Glauber's salt. Figure 1 shows the comparative volume of each material for the same amount of heat storage, based on the example in Worksheet I.
Storage material Bulk density Specific heat Latent heat
----------------------------------------------------------------------------
Rock 100 lb./cu.ft. 0.2 BTU/lb.°F ---------------
Water 62.4 lb./cu.ft. 1 BTU/lb.°F ---------------
Glauber's salt 56 lb./cu.ft. 0.5 BTU/lb.°F 108 BTU/lb. at 90°F
(phase change (including heat below 90°F
temp., 90°F) exchanger) 0.8 BTU/lb.°F
above 90°F
-----------------------------------------------------------------------------
Example: Assume your home has a heating requirement (estimated heat loss) of 15,000 BTU per hour, and you want your solar heating system to have a 3-day storage reserve. What would be your required storage capacity using rock, water or Glauber's salt as storage material?
Our Your
Items and calculations example situation
1. Volume required using ROCK as storage medium
a. Heating requirement of building: Estimated heat loss (see discussion above). = 15,000 BTU/hr ___________
b. Hours per day: 24. = 24 hr/day ___________
c. Desired storage reserve: Average 3-5 days (see discussion above). = 3 days ___________
d. Total heat needed: Step 1.a (15,000 BTU/hr.) x Step 1.b (24 hr./day) x Step 1.c
(3 days). = 1,080,000 BTU ___________
e. Bulk density of storage material: From Table 1. = 100 lb/cu.ft ___________
f. Specific heat of storage material: From Table 1. = .2 BTU/lb°F ___________
g. Temperature range over which heat is stored: Average 50-75°F (see
discussion above). = 50°F -----------
h. Heat per cu ft. of storage material: Step 1.e (100 lb/cu.ft.) x Step 1.f.
(.2 BTU/lb°F) x Step 1.g (50°F). = 1000 BTU/cu.ft ___________
i. Storage volume required using rock: Step 1.d (1,080,000 BTU) ÷ Step 1.h
(1000 BTU/cu.ft). = 1080 cu.ft ____________
2. Volume required using WATER as storage medium
a. Total heat needed: Same as Steps 1.a thru 1.d. = 1,080,000 cu.ft ___________
b. Bulk density of storage material: From Table 1. = 62.4 lb/cu.ft ___________
c. Specific heat of storage material: From Table 1. = 1 BTU/lb.°F ___________
d. Temperature range over which heat is stored: Same as Step 1.g. = 50°F ___________
e.Heat per cu. ft. of storage material: Step 2.b (62.4 lb./cu.ft.) x Step 2.c
(1 BTU/lb°F) x Step 2.d (50°F). = 3120 BTU/cu.ft __________
f. Storage volume required using water: Step 2.a (1,080,000 BTU) ÷ Step 2.e
(3120 BTU/cu.ft.). = 346 cu.ft ___________
3. Volume required using GLAUBER's SALT as storage medium
a. Total heat needed: Same as Steps 1.a thru 1.d. = 1,080,000 BTU ___________
b. Bulk density of storage material: From Table 1. = 56 lb/cu.ft ___________
c Latent heat of storage material: From Table 1. = 108 BTU/lb ___________
d. Specific heat of storage material: From Table 1.
*Above phase change temperature = .8 BTU/lb°F ___________
**Below phase change temperature = .5 BTU/lb°F ___________
e. Temperature differences between phase change (90°F) and storage
maximum (130°F) and minimum (80°F): See temperature-range discussion
above.
*Temperature difference above phase change = 40°F ___________
**Temperature difference below phase change = 10°F ___________
f. Heat per lb. of storage material: Step 3.c + (Step3.d* x Step 3.e*) + (Step 3.d**
x Step 3.e**). Example: 108 BTU/lb. + (.8 BTU/lb.°F x 40°F) + (.5 BTU/lb°F x
10F) = 108 BTU/lb. + 32 BTU/lb. + 5 BTU/lb. = 145 BTU/lb ___________
g. Heat per cu. ft. of storage material: Step 3.b (56 lb./cu.ft) x
Step 3.f (145 BTU/lb.). = 8120 BTU/cu.ft ___________
h. Storage volume required using Glauber's salt: Step 3.a (1,080,000 BTU)÷
Step 3.g (8120 BTU/cu.ft.). = 133 cu.ft ___________
Generally, for residential heating purposes, the storage can be contained within the house itself. Since it is heavy. the best location is in the basement or lowest level--and on concrete. not wooden supporting members. An interior storage facility should have some insulation, especially it the storage is charged during the summer. However, it need not be as heavily insulated as outdoor storage since the heat losses go directly to heating the house.
The storage can also be located exterior to the home either in the ground or in an unheated building. provided it is well insulated. Dry, well-drained soil acts as suitable insulation it the storage is buried outside; underground storage also permits more usable living space in the home.
The importance of storage facility configuration depends on the storage material used. Liquid storages are usually kept in a single large tank. Use of several smaller tanks would allow temperature maximization in a smaller volume, rather than having to heat the total volume of a single tank. However, because of the cost of multiple tanks and associated valving problems, and because there is significant vertical temperature stratification within a water tank, the recommended procedure is to use a single tank and take off the water at the top where it is warmest.
The effectiveness of rock storage is very dependent on configuration. The major concern in designing a rock storage facility is to minimize pressure drop in air flow through the storage. In general, the shorter a distance the air has to travel and the lower the air flow, the less the pressure drop will be.
The minimum length necessary for adequate heat transfer within the storage depends on air flow rate, heat transfer coefficient of air to rock, and the cross-sectional area. Under normal operating conditions, this minimum length is quite small. Therefore, the shorter the storage facility can be (within reason), the lower the operating cost. In general, an air flow of 20-30 feet per minute is desirable. The storage area can roughly be determined by dividing the total air flow from the collector (in cubic feed per minute) by the velocity (in feet per minute).
While air can be blown through the rock bed horizontally, the most efficient system is one designed for vertical airflow. The hot air from the collector is blown in the top, and the cool air is returned to the collector from the bottom. When heat is required to heat the room the airflow is reversed.
Since a building's heating requirement dictates the amount of solar heat that must be collected and stored, lowering that requirement will likewise decrease the collector area and storage capacity needed. Usually, the cheapest way to reduce heat loss is through proper insulation. In fact, the money saved by needing less storage space, storage material and collector area often more than pays for the extra insulation.
Just how much the addition of insulation can cut the cost of a solar heating system depends on a number of factors, such as the building's structural soundness, present insulation level, heat storage material to be used, etc. But the savings could be significant, as the example in Worksheet II shows. Use the worksheet to determine heating requirements and subsequent collection-storage system volume and costs at your present level of insulation and then at "proper" levels. In general, a storage unit should be insulated to a value of R-11 if in a heated area and to an R-30 if in an unheated area.
If the predicted solar energy-related construction "boom" indeed becomes a reality, there is certain to spring up some fly-by-night companies that will try to take advantage of consumer "ignorance concerning solar heat storage systems and materials. To protect yourself from these firms, as well as have a basis for making wise choices, follow this suggested procedure:
Single copies of the following Purdue Extension publications dealing with solar heating and energy conservation are available to Indiana residents from their County Extension Office or by writing to Media Distribution Center, 301 South Second Street, Lafayette, Indiana 47901-1232.
Solar Heating for Home, Farm and Small Business (AE-88)
Example: A typical square two-story home. with a roof surface area of 1267 square feet and a wall surface area of 2400 square feet is to be solar heated. It presently has as insulation only 6 inches of fiberglass (conductance value .053 BTU/hr.-°F-sq.ft. in the roof and 1 inch of fiberboard (conductance value .33 BTU/hr.-°F-sq. ft.) in the walls. Inside temperature will be held at 70°F: expected outside low temperature is 10°F. Should the owner design the air collector and Glauber's salt storage system for the home~s present heating requirement. or would it pay to add another 6 inches of insulation in the roof and 3 1/2 inches in the walls?
Our Your
Items and calculations example situation
1.Heating requirement of building with present insulation
a. Difference between inside and outside temperature: From example above
(70°F - 10°F). = 60°F _____________
b. Roof and wall surface areas; From example above.
*Root area = 1267 sq.ft _____________
**wall area = 2400 sq.ft _____________
c. Conductance value for present type and thickness of insulation:
Check with building material dealer. (Example: roof, 6 in.
fiberglass; wall, 1 in. fiberboard).
*Roof insulation = .053 BTU/hr-
°F-sq.ft _____________
**Wall insulation = .33 BTU/hr-
°F-sq.ft _____________
d. Heat loss from roof: Step 1.a (60°F) x Step 1.b* (1267 sq.ft.)
x Step 1.c* (.053 - BTU/hr-°F-sq.ft.). = 4029 BTU/hr ______________
e. Heat loss from walls: Step 1.a (60°F) x Step 1.b* (2400 sq.ft.) x
Step 1.c** (.33 BTU/hr.-°F-sq.ft.). = 47,520 BTU/hr ______________
f.Total present heating requirement: Step 1.d (4029 BTU/hr.) + Step 1.e
(47,520 BTU/hr). = 51,549 BTU/hr ______________
2.Amount and cost of storage material to meet present heating requirement
a. Hours per day: 24. = 24 hr/day _____________
b. Desired heat storage reserve: Avg. 3-5 days. = 3 days _____________
c. Heat storage capacity of storage material: For Glauber's salt,
see Worksheet I, Step 3.f
d. Per unit cost of storage material: Check with supplier. = $.25/lb _____________
e. Total storage material needed: (Step 1.f x Step 2.a x Step 2.b) ÷ Step 2.c.
Example: (51,549 BTU/hr. x 24 hr./day x 3 days) ÷ 145 BTU/lb.
= 3,711,526 BTU ÷ 145 BTU/lb. = 25,597 lb. _____________
f.Total cost of storage material needed: Step 2.e. (25,597 lb.) x Step 2.d
($.25/lb.). = $6399 ______________
3. Size and cost of collector to meet present heating requirement
a. Desired heating requirement collection capacity: Average 2 days. = 2 days ______________
b. Radiation value for collector: Check with supplier. = 1000 BTU/sq.ft ______________
c. Per sq. ft. cost of collector: Check with supplier. = $1.00/sq.ft ______________
d. Total collector area needed: (Step 1.f x Step 2.a x Step 3.a) ÷ Step 3.b.
Example: (51,549 BTU/hr. x 24 hr./day x 2 days) ÷ 1000 BTU/sq.ft
= 2,474,352 BTU ÷ 1000 BTU/sq.ft. = 2474 sq.ft ______________
e. Total cost of collector: Step 3.d (2474 sq.ft.) x
Step 3.c ($1.00/sq.ft.). = $2474 ______________
4.Heating requirement of building with added Insulation
a. Conductance value for present + added insulation: Step 1.c + added
insulation. (Example: roof 6 in fiberglass + 6 in. styrofoam; wall 1 in.
fiberboard + 3-1/2 in, styrofoam
*Root insulation = .026 BTU/hr- ______________
°F-sq.ft
**Wall insulation = .071 BTU/hr- ______________
°F-sq.ft
b. Heat loss from roof: Step 1.a (60 °F. x Step 1.b* (1267 sq.ft.)
x Step 4.a* (.026 BTU/hr-°F-sq.ft) = 1977 BTU/hr ______________
c. Heat loss from walls: Step 1.a (60°F) x Step 1.b** (2400 sq. ft)
x Step 4.a** (.071 BTU/hr)-°F-sq.ft). = 10,224 BTU/hr ______________
d. Total heating requirement with added insulation: Step 4.b (1977 BTU./hr) +
Step 4.c (10,224 BTU/hr) = 12,201 BTU/hr _____________
5.Amount and cost of storage material to meet "added insulation"
heating requirement
a. Total storage material needed:(Step 4.d x Step 2.a x Step 2.b) ÷ Step 2.c
Example: (12,201 BTU/hr x 24hr/day x 3 days ÷ 145 BTU/sq.ft =
878,472 BTU ÷ 145 BTU/lb = 6058 lb _____________
b. Total cost of storage material needed:
Step 5.a (6058 lb.) x Step 2.d ($.25/lb) = $1515 _____________
6.Size and cost of collector to meet "added insulation" heating
requirement
a. Total collector area needed: (Step 4.d x Step 2.a x Step 3.a) ÷ Step 3.b.
Example: (12,201 BTU/hr. x 24 hr/day x 2 days) - 1000 BTU/sq.ft. =
585,648 BTU ÷ 1000 BTU/sq.ft. = 586 sq.ft ______________
b. Total cost of collector:
Step 6.a. (586 sq. ft.) x Step 3.c ($1.00/sq. ft.). = $586 ______________
7. Heat system cost savings by adding insulation
a. Per-unit cost of insulation: Check with supplier. Example: 6 in. and 3-1/2 in.
mats.
*6-in. mats = $.20/sq.ft ______________
**3-1/2 in mats = $.12/sq.ft ______________
b. Cost of added insulation: (Step 1.b* x Step 7.a*) + (Step 1.b** x Step 7.a**).
Example: (1267 sq.ft. x $.20/sq.ft.) + (2400 sq.ft. x $.12/sq.ft.)
= $253 +$288. = $541 ______________
c. Total cost of heat system with present insulation: Step 2.f ($6399) + Step 3.e
($2474). = $8823 ______________
d. Total cost of heat system with added insulation: Step 5.b ($1515) + Step 6.b
($586) + Step 7.b ($541). = $2642 ______________
e. "Savings" realized by insulating: Step 7.c ($8873) -
Step 7.d ($2642). = $6231 ______________
New 9/78
Cooperative Extension work in Agriculture and Home Economics, state of Indiana, Purdue University, and U.S. Department of Agriculture Cooperating; H.A. Wadsworth, Director, West Lafayette, IN. Issued in furtherance of the acts of May 8 and June 30, 1914. The Cooperative Extension Service of Purdue University is an affirmative action/equal opportunity institution.