
NCH-46

H. D. Chapman, Louisiana State University
M. Sumner University of Georgia
T. Peck, University of Illinois
Interest in plant analysis as a crop management tool has been stimulated in recent years by increased use of scouting programs and crop consultants and by a higher level of sophistication among farmers themselves. In addition, narrowing profit margins and the continual pursuit of higher yields has spurred this interest.
The information provided through plant analysis helps farmers with decisions on fertilizer effectiveness, the need for additional nutrients, and planning fertilizer programs for future years. If used properly, plant analysis can be an important guide to efficient crop production because it provides a nutritional profile of the growing plant.
The objective of this publication is to explore the use and limitations of plant analysis in evaluating soil fertility programs for corn.
Plants require 16 elements for normal vegetative growth and reproduction. Different amounts of each element are required by different plant species. Plant growth is restricted when: 1) not enough of one or more elements are present; 2) too much of one or more elements are present, including toxic levels of nonessential elements such as aluminum, arsenic, selenium or sodium; or 3) the levels of one or more elements are adequate but out of balance with other elements.
The first result of nutrient deficiency, toxicity, or imbalance is a reduction in plant growth. If the condition persists, visible symptoms of deficiency or toxicity appear, and plant yield is reduced. "Hidden hunger" is a nutrient deficiency or imbalance not expressed in visible symptoms, but yield is restricted nevertheless.
Plant analysis is the quantitative determination of the elements in plant tissue. Carbon, hydrogen, and oxygen are not analyzed routinely because they come from air or water and virtually never limit plant growth. Chlorine is normally sufficient under field conditions, but it may become excessive in saline soils. It is usually analyzed in special cases only. Similarly, molybdenum deficiency or toxicity is rare, and this element is not analyzed routinely. Thus, plant analysis usually refers to analysis of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), and boron (B). Aluminum (Al) and sodium (Na) are sometimes included even though they are not essential elements. Aluminum can be toxic in acid soils, and sodium improves the quality of some crops such as beets and celery.
Plant analysis is distinguished from tissue testing in that it is a quantitative laboratory analysis; whereas tissue testing refers to semi-quantitative "quick" tests of plant sap carried out in the field for trouble-shooting purposes. Plant analysis is unique from other crop diagnostic tests in that it gives an overall picture of the nutrient levels within the plant at the time the sample was taken. Its use is based on the principle that the nutrient level present is a result of all factors affecting the growth of the plant.
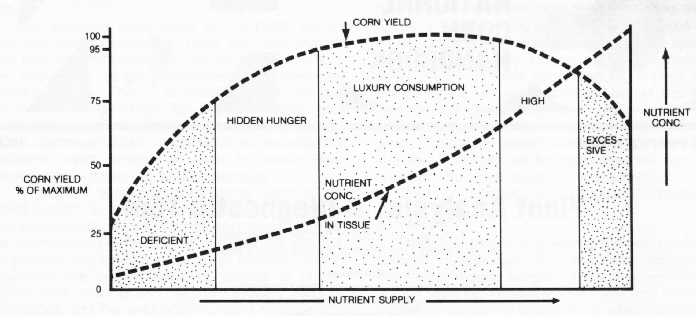
The general relationship between nutrient level and crop growth is shown in Figure 1. When a nutrient is deficient, addition of that nutrient results in increased crop growth and usually an increase in the concentration of that element in the plant. As the level of the deficient nutrient increases, crop growth increases until some maximum yield is reached. Further additions of the element will cause the concentration of that element in the plant to rise more rapidly because it is not being diluted by added dry matter accumulation. Eventually, toxicity of that element may occur.
Plant analysis has proven useful in confirming nutrient deficiencies, toxicities or imbalances, identifying "hidden hunger," evaluating fertilizer programs, determining the availability of elements not tested for by other methods, and studying interactions among nutrients.
Determining nutritional problems. Plant analysis defines nutrient problems more precisely than does an examination of deficiency symptoms, soil tests, or quick tissue tests. In addition to confirming suspected deficiencies, plant analysis can also detect toxicities or hidden deficiencies where visible symptoms are not manifest. One of the major uses of plant analysis is troubleshooting crop problems. Farmers seem to have confidence in the technology associated with plant analysis more so than with visual inspection and diagnosis. The second most common use is crop monitoring to evaluate potential nutritional problems while they can still be corrected or so they can be avoided in subsequent seasons.
Evaluating fertilizer programs. Adding fertilizer to the soil is no guarantee that plants will benefit from it. The form of the fertilizer might make it unavailable to plants, or it might react with the soil to form unavailable compounds. Soil scientists use plant analysis to study element uptake from fertilizer and to evaluate different methods and times of fertilizer application. Farmers can also use plant analysis to determine whether their fertilizer program is performing according to expectations.
Determining nutrient availability where soil tests are not available. Most laboratories test soils routinely for lime needs, phosphorus, and potassium. Some have optional tests for calcium, magnesium, and some of the minor elements. However, reliable soil tests have not been developed for all of the elements. A test for iron developed in one state, for example, is not applicable to the soils of another state until the test has been calibrated for the soils in that state. Plant analysis can be particularly advantageous in determining the availability of nutrients for which there are no reliable soil tests, or for those areas where soil test calibration has not been done.
Deficiencies of most micronutrients and sulfur are identified more accurately by plant analysis than by soil test. The soil test commonly used for sulfur, for example, measures only the amount of sulfate-sulfur present in the sampled area. It does not include possible contributions from other sources such as rainfall. A high sulfur soil test indicates adequate sulfur is present, but a low test may mean either the sulfur is not there or it was not measured. Plant analysis gives an accounting of the sulfur available to the plant.
Studying nutrient interactions. Plant analysis frequently reveals relationships among essential elements. While plant physiologists sometimes make these interactions a deliberate study, more often they discover these relationships when they summarize results of many plant analyses. This use of plant analysis is important for research but beyond the scope of "routine" use and will not be discussed here.
Sometimes adequate nutrient levels may be present in the soil, but because of other problems such as insect feeding, root damage, and too much or too little moisture, inadequate amounts of nutrients get into the plant. Plant analysis along with soil tests can help pinpoint the problem. Quite often the two techniques should be used together; for example, plant analysis of corn earleaf samples from central Wisconsin may show high levels of Mn present, but the soil analysis identifies the real problem as one of very acid soils.
Soil tests normally are calibrated for the average depth of plowing. If a subsoil is high in a particular nutrient, the subsoil contribution will go undetected unless a subsoil sample is also analyzed. A plant analysis will not tell how much of the nutrients in the plant came from the subsoil, but it will measure the integrated effect of the entire root volume. A soil sample typically consists of 5 to 10 cores of soil to tillage depth from a 5- to 20-acre field. A single corn plant, on the other hand, extracts nutrients from several cubic feet of soil.
Interpretation difficulties. In general, good relationships can be developed between soil nutrient supply, nutrient levels in the plant, and crop yield for a given location in any one year. However, differences in location, variety, time, and management often cause variations in these relationships and make them difficult to interpret. Nutrient levels in plants differ depending on the plant part sampled, stage of maturity, hybrid, and climatic conditions. Therefore, interpretations of plant analysis must take these factors into consideration. For this reason, most plant analysis interpretations are based on a specific plant part sampled at a definite stage of development. Greater detail on plant sampling for tissue analysis is in NCH-15.
For corn, the ear leaf at silking is most commonly used for analysis. In most situations, this is too late for remedial treatment if some is needed. The results of the analysis, then, can only be used to forestall future problems. In many cases, however, it is possible to identify nutrient disorders at an earlier stage of crop development if plants from a normal growing field at the same growth stage are also analyzed for comparison. The normal/abnormal comparison is often essential since sampling the entire plant tends to mask the differences in key parts of the plant. The plant must also be sufficiently mature to have developed a spread in concentrations.
Interrelationship of other factors. Martin and Matocha (1973) stated that "the basic principle of the use of plant analysis is that the chemical composition of the plant reflects its nutrient supply in relation to growth." They caution, however, that "the chemical composition of any plant is a `result' of the interaction of nutrient supply and plant growth. Any factor that limits growth. ..may cause other nutrients to accumulate in the plant." They point out that in using plant analysis as a diagnostic tool, "we are in effect attempting to infer a cause and effect relationship from two results (yield and nutrient concentration), either of which may have been brought out by some other factor." Thus, restricted root growth due to compacted soil layers or cold weather can result in reduced nutrient uptake even though the nutrient supply in the soil would be considered adequate under normal conditions.
Progressive deficiencies. Another limitation of plant analysis is that it usually detects only the one element that inhibits plant growth the most. Rarely are two or more elements acutely deficient at the same time. A corn plant, for example, may be deficient in K; but, because K is limiting growth, there may be sufficient P for the reduced amount of dry-matter production even if soil P is low. When K is added as a remedial treatment, dry-matter production increases sharply; then P becomes deficient. Nitrogen stress, on the other hand, can limit the uptake of phosphorus and some of the micronutrients to the extent that they appear to be "low."
Sample contamination. Contamination of a plant sample with soil particles or pesticide residue can lead to erroneously high results for iron, aluminum, manganese, zinc, or copper. Washing the sample to remove contamination can introduce other contaminants if a detergent or tap water are used. Appreciable potassium can be lost by washing. These problems are discussed further in NCH-15.
Sample deterioration. Decomposition of a plant sample before it reaches the laboratory will result in a loss of carbon (as CO2 through respiration and microbial activity) and the concomitant concentration of most other elements, thereby giving erroneously high readings. This can be prevented by refrigerating the sample until it is delivered to the lab, avoiding weekends if the sample is mailed, or by partially drying the sample before shipment. Solar drying to 15 to 20% moisture prior to shipment will not only eliminate the likelihood of sample spoilage but will reduce shipping costs as well. Brief drying in a microwave oven to about 10 to 15% moisture will halt enzymatic activity, but care must be taken to avoid over-drying the sample.
Critical value and sufficiency range approaches. For most diagnostic purposes, plant analyses are interpreted on the basis of "critical levels" for each nutrient. The critical level has been defined in several ways by different persons. Jones and Eck (1973) define it as "that concentration below which yields decrease or deficiency symptoms appear." For many nutrients, yield decreases even before visible deficiency symptoms are observed. Because the exact concentration of a nutrient below which yields decline is difficult to determine precisely, some define the critical level as the nutrient concentration at 90 or 95% of maximum yield.
The nutrient composition of a plant changes as the plant matures and with the portion of the plant sampled; therefore, critical levels are defined for a specific plant part at a specified stage of maturity. For corn, the ear leaf from tasselling to silking is most commonly used. For most crops, there is an optimal range of concentration over which yield will be maximized rather than a single point. If possible, one would attempt to supply nutrients at the lowest level which still provides top yields, but because of the many factors affecting yields and concentrations, this point is difficult to identify. Growers, therefore, usually strive for operating in the sufficiency range.
In the deficient range, visible nutrient deficiency symptoms are evident, and crop yield is less than 75% of maximum. In the low range, there are no clear-cut deficiency symptoms, yet responses to additions of the low nutrient are likely. The sufficiency range represents the yield plateau. The nutrient concentration increases more rapidly as nutrient supply increases because there is no further increase in dry matter production to "dilute" the additional nutrient. Most nutrients have fairly broad sufficiency ranges. The lower end of the sufficiency range (or the upper end of the low range) represents the critical range. In the "high" range the plant takes in more of the nutrient than is required for maximum production. This range is sometimes referred to as the zone of "luxury consumption."
If the nutrient supply is increased sufficiently, yields decline either because of an imbalance with other plant nutrients or direct toxic effects of the excessive nutrient. Phosphorus, for example, at high levels can suppress the uptake of copper and zinc and be out of balance with respect to nitrogen or potassium, but it is rarely toxic per Se. Boron, on the other hand, can easily become toxic to corn if misapplied.
Melsted et al. (1969) point out that critical levels of plant nutrients "can seldom be derived through a single carefully designed experiment." More typically, results of many experiments over a number of years and at various locations are averaged. Critical levels were published by Melsted et al. (1969) and sufficiency ranges were published by Jones (1967) and Neubert et al. (1969), and they are given in Table 1. Also included are the sufficiency ranges used by the Soil and Plant Analysis Lab, University of Wisconsin-Madison. These were compiled from a number of sources, including Jones (1967), Chapman (1966), and others. Agreement is remarkably close considering the variety of sources from which the data were derived.
Nutrient ranges for corn representing deficient, low, sufficient, high, and excessive concentrations used by the Soil & Plant Analysis Lab, UW-Madison, are given in Table 2. For some nutrients, excessive nutrient levels have not been well-defined. These ranges are useful guidelines for interpreting plant analyses, but they must not be used dogmatically. Knowledge of hybrid requirements, unusual soil or climatic conditions, or other extenuating information should be taken into account.
As plant analysis becomes more popular as a diagnostic tool, the need for earlier sampling and analysis intensifies so problems can be corrected before serious yield loss occurs. Plant analyses interpretations at early growth stages would require that critical levels be established for those stages. Unfortunately, limited data are available on critical nutrient concentrations for very young plants. Lockman (personal communication) has developed sufficiency ranges for whole corn plants 24 to 45 days after planting as well as ranges for more mature corn (see Table 3). Nutrient uptake and dry matter accumulation are generally rapid during early stages of growth. Consequently, nutrient concentrations may be expected to vary considerably with maturity, as reflected in the rather wide sufficiency range for the mobile elements. The concentration of these elements (N, P, K, Mg) in whole plant tissue is appreciable higher at 24 to 45 days than is their concentration in earleaf tissue. A variation of a few days in sampling time at this stage of growth would be more critical than at silking stage.
Multiple regression approach. Modern analytical multiple-element analysis gives results which lend themselves to multiple-regression analysis for interpretation. Greater information is gained since additional complex interactions can be evaluated with respect to yields. Although some work in this manner has been done, particularly for N, P, and K, this type of interpretation has not been incorporated into routine plant analysis programs.
Nutrient ratio approach. A variation of the multiple regression approach recently developed is the Diagnosis and Recommendations Integrated System (DRIS) developed by Beaufils (1971,1973) and introduced in this country by Sumner (1977a, 1977b, 1979). As originally developed, as many yield determining factors as are capable of quantitative or qualitative expression are considered simultaneously in making a diagnosis, and recommendations are made from that diagnosis. These factors include not only soil and plant analysis data but also information pertaining to climate, insects, disease, varieties, etc.
The DRIS approach to interpreting the results of plant analysis involves the analysis of thousands of samples of a specific crop. The samples are divided into high and low yielding populations, and the analytical results from each population are studied to determine what criteria can be used to distinguish between the high and low yielding populations. As it turns out, ratios of plant nutrient concentrations have given better results than simple concentrations alone. The ratios corresponding to the high yielding population (norms) are then compared to the ratios present in the sample being analyzed. A ratio of plant nutrient concentrations by itself cannot be used to diagnose plant problems, but combinations of different nutrient ratios can be combined mathematically to determine what nutrients are most likely to limit yield. The results of such calculations are the "DRIS Indices."
Although finer tuning may be possible, DRIS indices are normally calibrated so that those within the range of about -10 or -15 to +10 or +15 are considered normal and in balance. A DRIS index between -25 and -15 indicates a likely deficiency. Values greater than +25 may be an indication of possible nutrient excess. The greater the magnitude of the nutrient index, either positive or negative, the more likely that element is out of balance in the plant.
Melsted Neubert UW Soil &
et al. Jones et al. Plant anal.
Nutrient (1969) (1967) (1969) Lab
-------------------------------------------------------------
N, % 3.00 2.76-3.50 2.60-4.00 2.76-3.75
P, % 0.25 0.25-0.40 0.25-0.50 0.25-0.50
K, % 1.90 1.71-2.50 1.70-3.00 1.75-2.75
Ca, % 0.40 0.21-1.00 0.21-1.00 0.30-0.60
Mg, % 0.25 0.21-0.60 0.31-0.50 0.16-0.40
-- --- 0.21-0.50 0.16-0.50
Zn,ppm 15 20-70 50-150 19-75
B,ppm 10 4-25 15-90 5-40
Mn, ppm 15 20-150 34-200 19-75
Fe, ppm 25 21-250 21-250 50-250
Cu,ppm 5 6-20 8-20 3-15
-------------------------------------------------------------
Nutrient Concentration in Tissue
Nutrient Deficient Low Sufficient High Excessive
-----------------------------------------------------------------------
N, % <1.75 1.76-2.76 2.76-3.75 >3.75 --
P, % <0.16 0.16-0.24 0.25-0.50 >0.50 --
K, % <1.25 1.25-1.74 1.75-2.75 >2.75
Ca,% <0.10 0.10-0.29 0.30-0.60 0.61-0.90 >0.90
Mg,% <0.10 0.10-0.15 0.16-0.40 >0.40 --
<0.10 0.10-0.15 0.16-0.50 >0.50 --
Zn,ppm < 12 12-18 19-75 76-150 >150
B, ppm <2.0 2.0-5.0 5.1-40.0 41-55 >55
Mn,ppm < 12 12-18 19-75 >75 --
Fe, ppm < 10 10-49 50-250 251-350 >350
Cu,ppm --- < 3 3-15 16-30 >30
-----------------------------------------------------------------------
<= "less than"
>= "more than"
Whole plant, 3rd leaf, Earleaf Earleaf Earleaf
Nutrient 2445 days1 45-80 days2 green silks3 brown silks4 mature5
---------------------------------------------------------------------------
N, % 4.0-5.0 3.5-4.5 3.0-4.0 2.8-3.5 2.5-3.5
.40-.60 .35-.50 .30-.45 .25-.40 .20-30
K, % 3.0-5.0 2.0-3.5 2.0-3.0 1.8-2.5 1.6-2.5
Ca, % .51-1.6 .20-.80 .20-1.0 .20-1.2 .20-1.5
Mg,% .30-.60 .20-60 .20-.80 .20-.80 .20-80
S, % .18-40 .18-40 .18-.40 .18-35 .16-.35
B, ppm 6-25 6-25 5-25 5-25 5-25
Cu,ppm 6-20 6-20 5-20 5-20 4-20
Fe, ppm 40-500 25-250 30-250 30-250 30-250
Mn,ppm 40-160 20-150 20-150 20-150 20-150
Zn, ppm 25-60 20-60 20-70 20-70 16-50
-------------------------------------------------------------------------
1 Seedlings 6 to 16 inches tall; 24 to 45 days after planting.
2 Third leaf from top; plants over 12 inches tall, before silking.
3 70 to 90 days after planting.
4 Grain in developing stage up to "roasting ear."
5 Poor stage-sample; grain in dough stage, beginning to dent.
The principal advantages of the DRIS system are that stage of maturity, plant part, and cultivar are less important than they are for the critical level or sufficiency range approaches to interpreting plant analyses. Thus, it is possible to sample corn at the knee-high stage, for example, rather than waiting for the silking stage. However, Sumner (personal communication) has determined recently that different norms should be used for whole plant samples than those use for leaf samples.
A problem encountered by some users of the DRIS system is a tendency to interpret the results too dogmatically. Some regard every negative index as representing a deficiency and pay no attention to positive indices. But because all scientific measurements are subject to some degree of uncertainty, both positive and negative indices must be evaluated, and the evaluations should not be made disregarding nutrient concentrations altogether.
Another limitation is that not all of the norms used to develop DRIS indices have been evaluated under field conditions. High yielding corn might be high in Ca, for example, because soil conditions required to obtain high yields coincidentally favored luxury consumption of Ca. The need for Ca is rarely evaluated under field conditions, this need being regarded as fulfilled by liming, although raising the pH of an acid soil provides many plant benefits in addition to supplying Ca.
The results of plant analysis alone cannot be used to make fertilizer recommendations. Although plant analysis can provide substantial additional information, plant samples should be accompanied by soil samples taken from the same area as the plants. If the plant and soil samples are taken from an abnormal area of a field, the results are applicable to that area only. Unless a field is sampled in detail, the soil sample accompanying a plant sample usually is not very representative of the entire field. Low or deficient values for either soil or plant analysis signal the need for such detailed soil sampling. Emergency recommendations for an abnormal area in a field can be made from soil and plant analyses, but field scale recommendations should be based on detailed soil sampling and analysis.
Plant analysis is a powerful tool for confirming nutrient deficiencies, toxicities and imbalances, identifying "hidden hunger," evaluating fertilizer programs, studying nutrient interactions, and determining the availability of elements for which reliable soil tests have not been developed. The results can be misleading, however, if initial plant sampling, handling, and analysis of the sample are faulty. Experience with interpreting the overall plant analysis report is essential because of the many interacting factors which influence the concentration of any one element in plant tissue. After assessing the status of each nutrient per Se, one needs to review possible causes of the effects observed. Thus, cropping history, sampling techniques, soil test data, and a knowledge of nutrient concentrations all need to be considered in the final diagnosis. If properly done, plant analysis can point the way toward more efficient use of fertilizer investments.
RR 4/91
Cooperative Extension Work in Agriculture and Home Economics, State of Indiana, Purdue University and U.S. Department of Agriculture Cooperating. H.A. Wadsworth, Director, West Lafayette, IN. Issued in furtherance of the Acts of May 8 and June 30, 1914. It is the policy of the Cooperative Extension Service of Purdue University that all persons shall have equal opportunity and access to our programs and facilities.