
WQ-12

Distillation is one of the oldest methods of water treatment and is still in use today, though not commonly as a home treatment method. It can effectively remove many contaminants from drinking water, including bacteria, inorganic and many organic compounds.
Your first step toward solving a suspected water quality problem is having your water analyzed by the local health department or a reputable laboratory. A water analysis not only verifies if a water quality problem exists, but is essential to determine the most appropriate solution to the problem. State or local health officials can interpret water analysis results. Some labora- tories may also provide this service.
Home water treatment should be considered only a temporary solution. The best solutions to a contaminated drinking water problem are to stop the practices causing the contamination or change water sources.
Distillation relies on evaporation to purify water. Contaminated water is heated to form steam. Inorganic compounds and large non-volatile organic molecules do not evaporate with the water and are left behind. The steam then cools and condenses to form purified water.
Distillation effectively removes inorganic compounds such as metals (lead), nitrate, and other nuisance particles such as iron and hardness from a con- taminated water supply. The boiling process also kills microorganisms such as bacteria and some viruses. Distillation removes oxygen and some trace metals from water. For this reason some people claim distilled water tastes flat.
Distillation's effectiveness in removing organic compounds varies, depending on such chemical characteristics of the organic compound as solubility and boiling point. Organic compounds with boiling points lower than the boiling point of water (ex. benzene and toluene) vaporize along with the water. These harmful compounds will recontaminate the purified product if not removed prior to condensation.
Distillation units, or stills, generally consist of a boiling chamber, where the water enters, is heated and vaporized; condensing coils or chamber, where the water is cooled and converted back to liquid water; and a storage tank for purified water. Figure 1 shows the parts and process of a distiller.
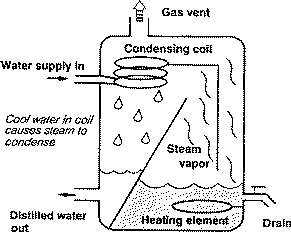
Distillation units are usually installed as point-of-use (POU) systems. They are generally placed at the kitchen faucet and used to purify water for drinking and cooking only. Size varies, depending on the amount of purified water they produce. Production rates range from 3 to 11 gallons per day. Home stills can be located on the counter or floor, or attached to the wall. Models can be fully or partially automated, or manual.
Some units have columns or volatile gas vents to eliminate organic chemicals with boiling points lower than water, thus ensuring uncontaminated water.
As with all home water treatment systems, distillation units require some level of regular maintenance to keep the unit operating properly. Uneva- porated pollutants left in the boiling chamber need to be regularly flushed to the septic or sewer system. Even with regular removal of the residual water containing unevaporated pollutants, a calcium and magnesium scale will collect at the bottom of the boiling chamber. The scale eventually needs to be removed, usually by hand scrubbing or by using acid.
Heating water to form steam requires energy. Which means the operating costs for distillation units are generally higher than those for other home water treatments. The production of heat from home distillation units can be an advantage in winter, but a disadvantage in summer.
Distillation units are generally expensive, ranging from $300 to $1200. Port- able units can be purchased for less than $200. (NOTE: Dollar values are provided as a rough guide to compare costs of different systems. Current prices are likely to be higher than those quoted.)
Certification of treatment products is available from independent testing laboratories, such as the National Sanitation Foundation (NSF). Results from NSF tests provide good measures of the effectiveness of devices designed to treat water for both esthetic and health reasons. The Water Quality Associa- tion (WQA) is a self-governing body of manufacturers and distributors. WQA offers voluntary validation programs to its members. Validation is less stringent than certification. Certification or validation does not ensure effective treatment; all systems must be designed for each particular situa- tion and maintained properly.
Treating drinking water by using home distillation units is one option avail- able if you have a water quality problem. Distillation effectively removes inorganic compounds, bacteria, particles and some organic contaminants. How- ever, other treatment methods may be better for these contaminants and more cost-effective as well. Distillation is not a very common method for home water treatment.
For further information on water quality contact your county Cooperative Extension office or local health department. The following bulletins in the WQ series may also be helpful:
- WQ 1 "Water Testing Laboratories"
- WQ 2 "What Is Ground Water?"
- WQ 3 "How to Take a Water Sample"
- WQ 4 "Why Test Your Water?"
- WQ 5 "Interpreting Water Test Results Part One: Inorganic Materials"
- WQ 6 "Buying Home Water Equipment"
- WQ 9 "Water Quality for Animals"
- WQ 10 "Wetlands and Water Quality"
- WQ 11 "Sulphur Water Control"
- WQ 13 "Home Water Treatment Using Activated Carbon"
- WQ 14 "Reverse Osmosis for Home Treatment of Drinking Water"
- WQ 16 "Bacterial Contamination of Household Water"
Kamrin, Michael, Nancy Hayden, Barry Christian, Dan Bennack and Frank D'Itri, WQ 22 "Distillation For Home Water Treatment," Cooperative Extension Service, Michigan State University, 1990.
*Reviewed and revised by Adel Pfeil, Extension Specialist, Department of Con- sumer Sciences and Retailing.
Editor: Cheri L. Janssen, Department of Agronomy
Cooperative Extension work in Agriculture and Home Economics, state of Indiana, Purdue University, and U.S. Department of Agriculture cooperating; H. A. Wadsworth, Director, West Lafayette, IN. Issued in furtherance of the acts of May 8 and June 30, 1914. The Cooperative Extension Service of Purdue University is an affirmative action/equal opportunity institution.